Abstract
[Background] Differentiation therapy with retinoic acid (RA) has remarkably improved the cure rate for acute promyelocytic leukemia (APL). RAs are known to have anti-leukemic effect in parts of non-APL acute myelocytic leukemia (AML) cells. It has been reported that AML patient with NPM1 or IDH1/2 mutation may have sensitivity to all-trans retinoic acid (ATRA) and the clinical study with combination of chemotherapy and ATRA is conducted in NPM1 mutated AML. In addition, ATRA and a FLT3 inhibitor, quizartinib, showed synergistic activity against FLT3-ITD positive AML patients' samples and their combination therapy improved the survival of AML xenograft mouse model. Moreover, it is also reported that the presence of super-enhancer at RARA gene locus is related to high RARA mRNA expression and sensitivity to tamibarotene. Another study showed that ATRA promote cancer apoptosis in the presence of Cellular Retinoic Acid-Binding Protein 1 (CRABP1) through G1 expansion. However, the efficacy of RAs and its mechanism in non-APL AML are still largely unknown. In this study, we evaluated anti-leukemic effect of RAs on primary AML cells and explored its predictive biomarkers for the purpose of optimization of therapy for non-APL AML.
[Method] We examined growth inhibitory and differentiation effects of ATRA and tamibarotene on primary AML cells in vitro. Primary AML cells were purified by density-based centrifugation and cultured with various concentrations of RAs in methylcellulose-based media including cytokines. IC50 values of RAs were calculated by luminescent cell viable assay on day7 and 14. Cell differentiation upon RAs treatment was also examined in these cells by morphology and expression of CD11b using flow cytometry analysis. We also evaluated the efficacy of combination therapy of RAs and several tyrosine kinase inhibitors, quizartinib, midstaurin and dasatinib, and assessed their synergistic effect by calculating combination index. The association of IC50 values of RAs with clinical characteristics, mRNA expressions of RARA and CRABP1, and genetic mutations were investigated.
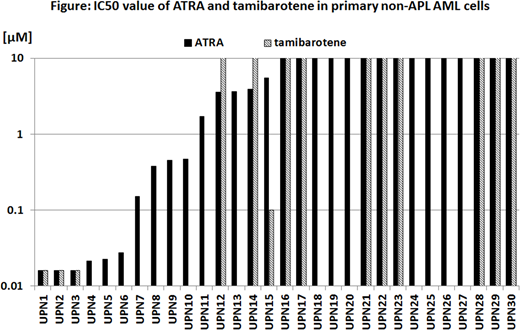
[Result] We analyzed total of 30 primary non-APL AML samples and IC50 values of ATRA and tamibarotene were lower than 5 µM in 14 of 30 (46.7 %) and 4 of 14 (28.6 %) AML cells, respectively. There were no significant difference in IC50 value of ATRA and tamibarotene by FAB subtype and chromosomal abnormalities. ATRA and tamibarotene induced differentiation in 10 of the 22 and 2 of the 9, respectively, and anti-leukemic effect of ATRA and tamibarotene were not associated with these differentiation statuses. IC50 value of ATRA and tamibarotene were significantly lower in ASXL1 (p=0.0071 and p=0.0012, respectively), SRSF2 (p=0.0101 and p=0.0113, respectively), and IDH2 (p=0.0295 and p=0.0044, respectively) mutated AML cells. FLT3-ITD mutation with high allelic ratio (>0.5) was associated with high IC50 value of ATRA (p=0.0116). Although NPM1 mutation was associated with higher IC50 value of ATRA, all of 4 patients with NPM1 mutation concurrently have FLT3-ITD mutations in this study. Other gene mutations were not correlated with RAs sensitivity. The expression of RARA mRNA weakly correlated with sensitivity of ATRA and tamibarotene (P=0.1126 and P=0.0711 respectively), but it was not significant.
[Conclusion] RAs were effective in almost half of non-APL AML cells in vitro and some AML cells were sensitive to ATRA without cell differentiation. Mutation in ASXL1, SRSF2, and IDH2 were identified as predictive factors for the RAs sensitivity, whereas the expression of RARA mRNA and other gene mutations were not correlated with RAs sensitivity.
Kiyoi:Takeda Pharmaceutical Co., Ltd.: Research Funding; Phizer Japan Inc.: Research Funding; Sumitomo Dainippon Pharma Co., Ltd.: Research Funding; Chugai Pharmaceutical Co., Ltd.: Research Funding; FUJIFILM Corporation: Research Funding; Kyowa Hakko Kirin Co., Ltd.: Research Funding; Sanofi K.K.: Research Funding; Otsuka Pharmaceutical Co., Ltd.: Research Funding; Novartis Pharma K.K.: Research Funding; Zenyaku Kogyo Co., Ltd.: Research Funding; Eisai Co., Ltd.: Research Funding; Bristol-Myers Squibb: Honoraria; Celgene Corporation: Research Funding; Astellas Pharma Inc.: Research Funding; Nippon Shinyaku Co., Ltd.: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal